 Laboratory Equipment
Laboratory Equipment
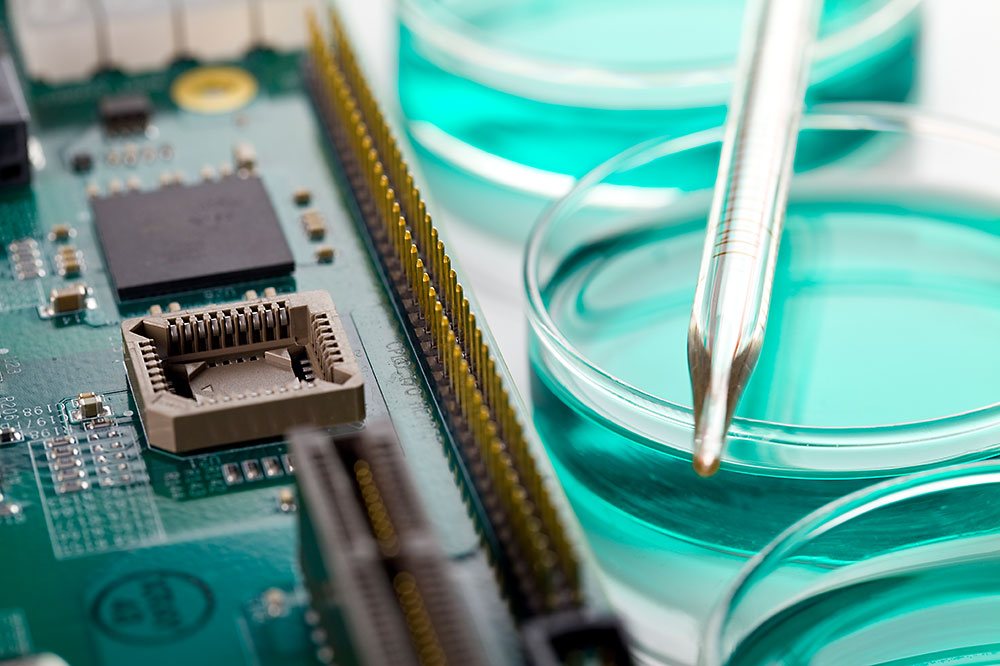
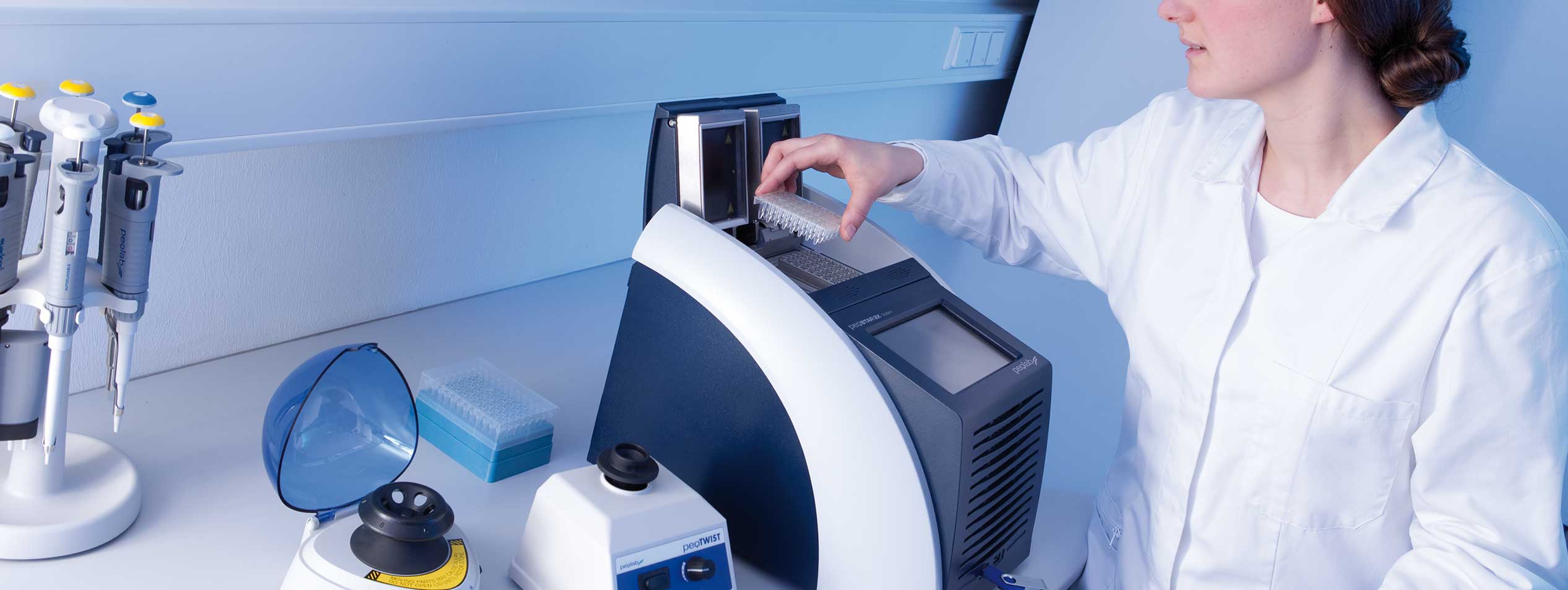
We develop, design and produce laboratory equipment for various research and diagnostic facilities. These are used in numerous applications in the life sciences sector and since many years belong to the permanent equipment of many laboratories.
You require development services for mechanics, electronics or special software in this field? We are a reliable partner for you in these areas. Our strength lies in fast and high-precision temperature control systems such as the proven thermal cycler "peqSTAR". To achieve this we rely on state-of-the-art development technologies such as the simulation of thermodynamic processes.
 Medical Technology
Medical Technology
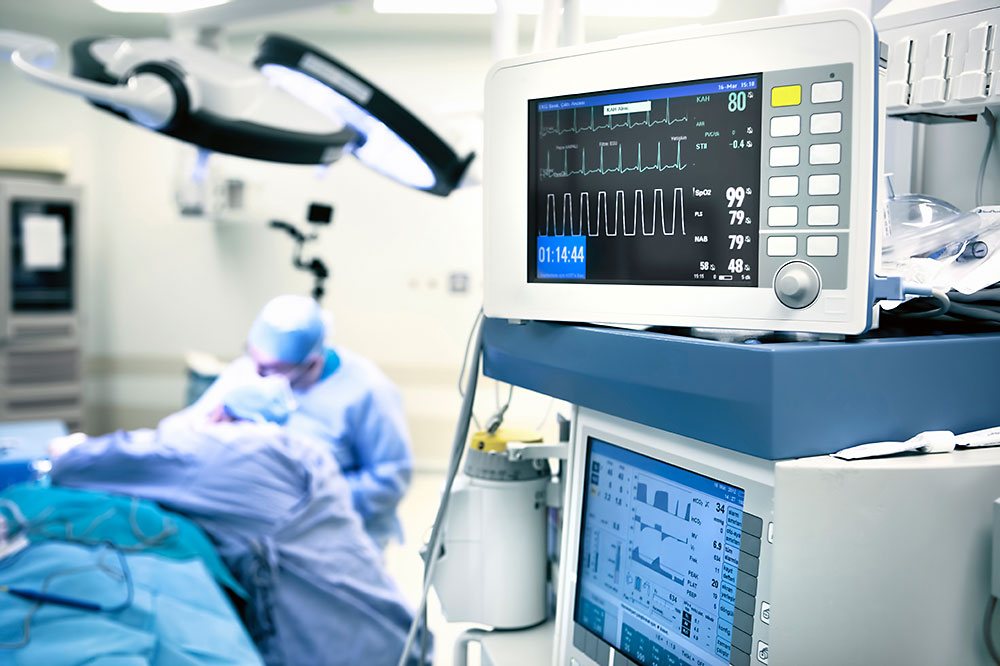
Medical technology, also known as "biomedical engineering", combines knowledge from the field of technology with the medical expertise of physicians to improve diagnosis, treatment and rehabilitation of sick people. For this sensitive area, we offer the development and production of medical systems and components with the close integration of products and services. For many years now, for example, we are manufacturing components for an eye diagnostic system. With our certification to ISO 9001 and ISO 13485, we are a powerful and competent partner at your side.
 Testing Technology
Testing Technology
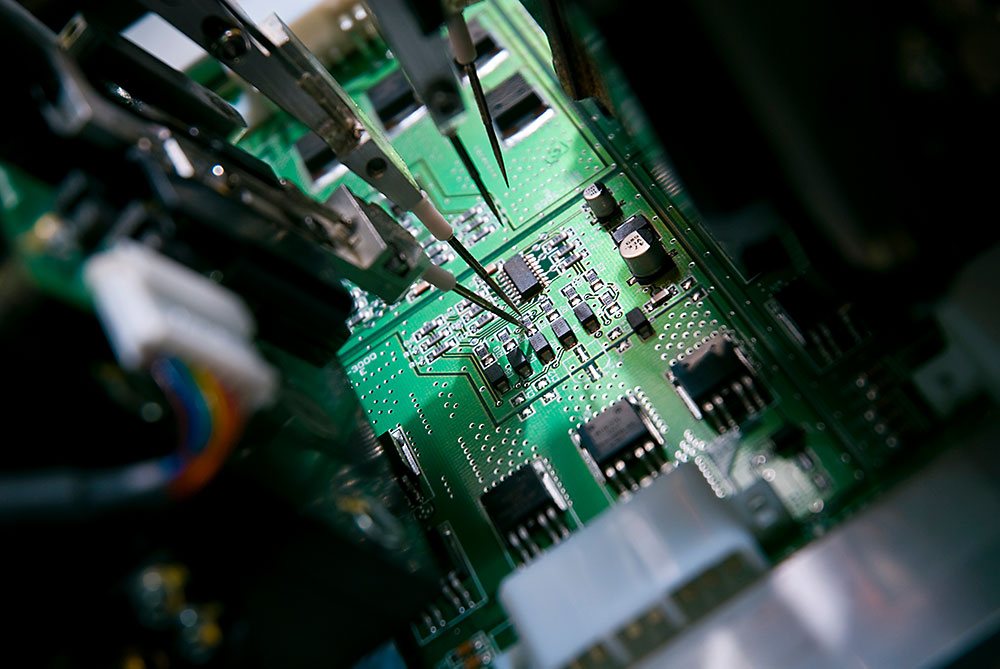
Testing is an essential part of the production process, as often extensive, safety-related functions are tested. In particular for the automotive industry, this aspect plays an important role, especially as harsh conditions prevail in the vehicle that affect all elements of the printed circuit board and automotive wiring. For many years we are delivering reliable electronic components for various highly complex test systems for printed circuit boards and test systems for the automotive electronics and wiring. The highest level of quality awareness is a given for us. The development of highly efficient testing technology for other applications is a commitment we gladly accept.
 Electronic Controls
Electronic Controls
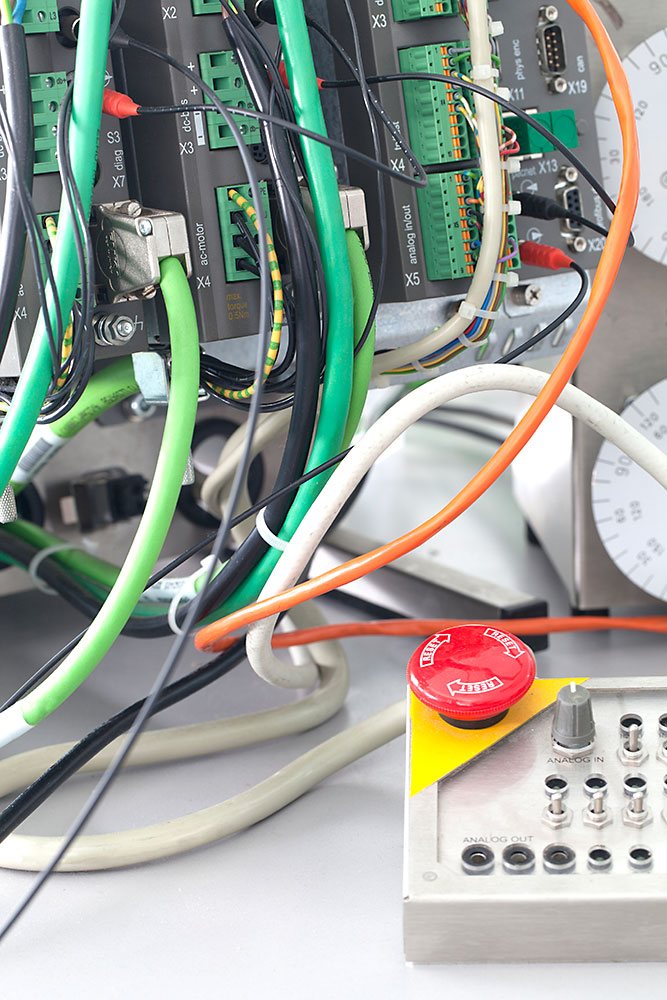
Measuring, controlling and regulation of different process variables with the most modern systems is a broad and challenging field in today's automation and production engineering.
We are experts in the development and manufacture of electronic control, measurement and control systems, ranging from mechanics, analog and digital electronics, software-implemented and control algorithms to modern graphical user interface with touch screen technology. Several successfully completed development projects confirm our qualification.



 Laboratory Equipment
Laboratory Equipment Medical Technology
Medical Technology Testing Technology
Testing Technology Electronic Controls
Electronic Controls 



